WCG eResearch Enterprise CTMS
An integrated platform designed to support all of your site’s clinical trials and research activities, from start-up through close-out.
Operational efficiency and consistency. Financial oversight. Interoperability across technologies. In today’s complicated clinical research environment, sites need increased financial visibility and strong operational oversight in addition to increased coordinator and participant support.
WCG eResearch Enterprise CTMS is a comprehensive and adaptable clinical trial management system designed to automate all administrative, financial, and research activities to help sites accelerate their research management with scalable growth and continuous improvement.
Our clients – including academic medical centers, hospitals, health systems, and independent sites – are more productive and efficient with WCG eResearch Enterprise CTMS.
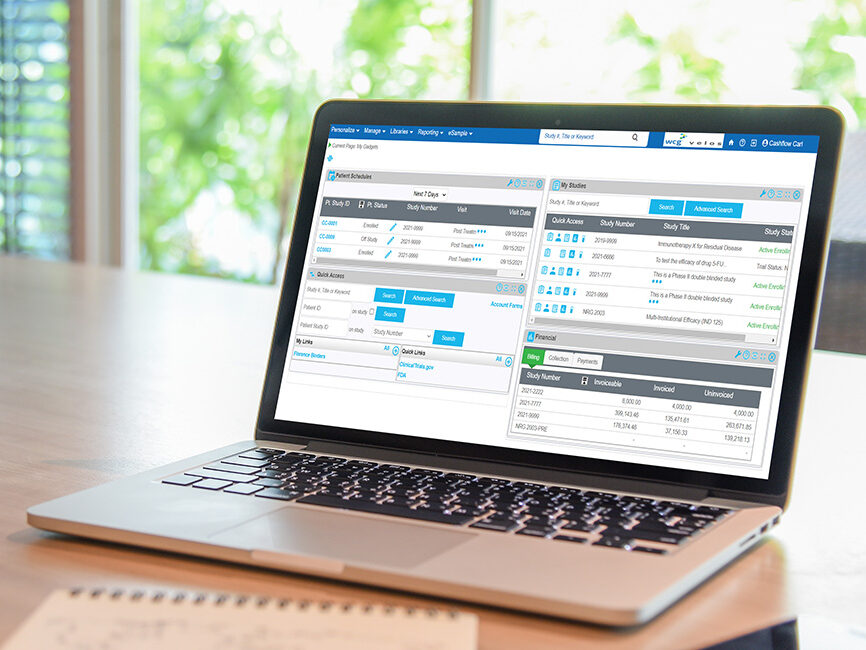
WCG eResearch Enterprise CTMS Supports Your Clinical Research Site With:
Integrated operations
Connect across technologies to provide information and reporting insights, enhance visibility within the organization, and increase end-to-end operational efficiency and consistency.
Financial and regulatory oversight
Efficiently evaluate study opportunities, ensuring fast turnaround for customer satisfaction and sponsor payment, while meeting compliance standards to manage risk, avoid penalties, and support audits.
Coordinator and participant-centricity
Effectively manage participants and visits with the included participant portal for remote participant engagement and self-reporting. Oversee numerous studies simultaneously while eliminating duplicate data entry and the need to interface with multiple, complex systems.
Key Features of eResearch Enterprise CTMS
Study Initiation
Study Management
Study Closeout

Study Setup
- Protocol Definition
- Study Calendar
- Document Storage
- Budget
- Coverage Analysis
- CRF Design
- Study Team
- Status Tracking
- Submission Review

Participant
Management
- Coordinator Portal
- Participant Profile
- Participant Status
- Consent
- Visit Tracking
- Deviation Tracking
- Participant Portal
Financial
Management
- Charge Master
- Milestones
- AR/AP
- Invoicing
- Payment
- Reconciliation
Clinical Data
Management
- Electronic Data Capture
- Case Report Forms (eCRFs)
- Data Monitoring
- Query Management
- Validations

Reporting &
Dashboards
- Standard Dynamic Reports
- Ad-Hoc Queries
- Study Dashboards
- Finance Dashboards
- Data Monitoring Dashboards
- Query Management Dashboards
- Actionable Intelligence
Real Results with WCG eResearch Enterprise CTMS
WCG eResearch Enterprise CTMS helps you ensure a single source of truth for coordinators as they access study documents and interface with EHRs and other enterprise systems. Your team will spend less time on administrative work and more time with participants, using our intuitive and dependable technology.
reduction in time spent on data entry
improvement in site financial results
system integrations, including 50+ with Epic
increase in participant retention rates
Customer Stories
Simplifying Clinical Research Processes Across a Large Academic Medical Center with WCG eResearch
- Improved financial transparency with coverage analysis documentation, calendar, budget, and milestone entry.
- Demonstrated increased efficiency and profitability with the integrated tracking of account receivables and invoices.
- Eliminated duplicate data sets across multiple departments.
There’s rarely a question we can’t answer with WCG. WCG eResearch has improved our institution’s research programs and operations, and as a result, improved the attractiveness of our site to sponsors and CROs.
– Director of Research Information Management, Large Academic Medical Center
Part of an Integrated Research Platform
WCG eSample
Manage all biospecimens for your research, or set up biobanks or biorepositories using one secure platform.
WCG eSample enables biobanks, biorepositories, and research sites to manage specimens of all types on one secure, centralized, and integrated platform.
WCG Participant Portal
Engage participants and facilitate research in remote settings.
WCG Participant Portal is designed to sustain and accelerate research even in remote settings, encouraging compliance and timely communication between participants and care providers.
WCG eResearch Enterprise CTMS FAQs
Schedule a demo of WCG eResearch CTMS
Learn how you can streamline your clinical trials and improve productivity. By completing this form, someone will reach out to you within 24 hours to set up a demo of our industry-leading CTMS technology.
Recent Insights

7 Key Considerations for Research Sites Contemplating Switching Their CTMS
Blog Posts
Clinical Trial Technologies for Sites: Building an Innovative Clinical Trial Technology Roadmap For Your Research Program
Videos